)
)
Research & Development
Innovation is in our DNA
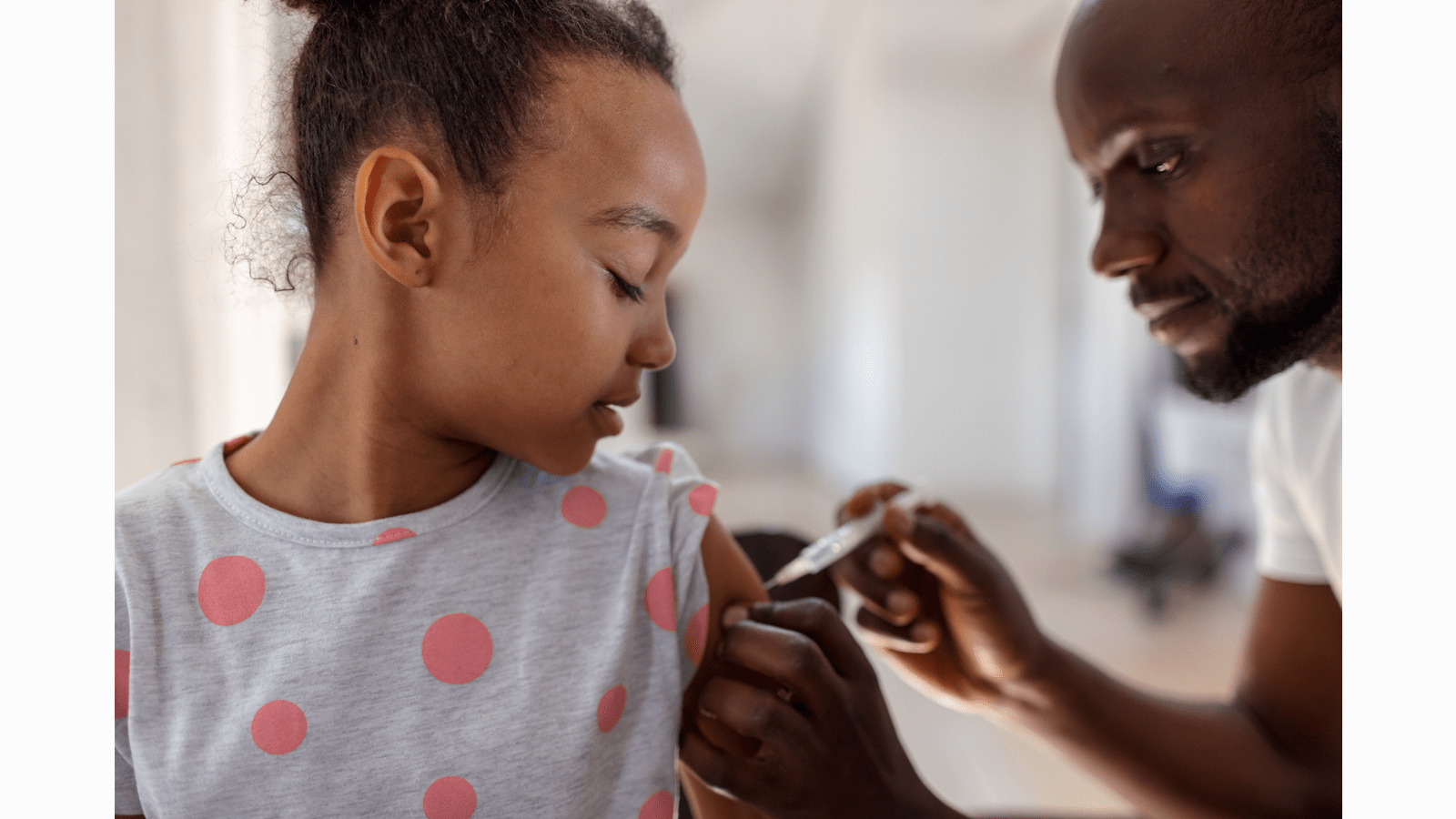
Investment in R&D is an important driver for CSL’s future growth. New and exciting opportunities allow us to address previously unmet patient needs and these continue to drive us each day. In addition, CSL continues to build on its capabilities across the R&D value chain, from discovery research to pharmacovigilance to its currently marketed therapies. Such proficiency is critical as R&D builds the novel and diverse pipeline of the future. We continue to make a balanced investment in the life cycle management and market development of existing products that bring short to mid-term commercial benefits, and we make strategic investments in longer-term, higher-risk and high-opportunity new product development activities.
-
US $1.2 billion
was invested in R&D in 2022/23
-
US $5.1 billion
in R&D investments in last 5 years
-
>2,000
Research & Development employees